44-DMAR/buy 44-dmar online
44-DMAR/buy 44-dmar online
44-DMAR/buy 44-dmar online . 4,4′-Dimethylaminorex (abbreviated as 4,4′-DMAR), sometimes referred to by the street name “Serotoni”, is a psychostimulant and entactogen designer drug related to aminorex, 4-methylaminorex, and pemoline.[2] It was first detected in the Netherlands in December 2012, and has been sold as a designer drug around Europe since mid-2013.
44-DMAR/buy 44-dmar online had been linked to at least 31 deaths in Hungary, Poland, and the UK by February 2014, mostly when consumed in combination with other drugs.[4] Nineteen deaths linked to 4,4′-DMAR were reported in Northern Ireland in the same time period.
44-DMAR/buy 44-dmar online acts as a potent and balanced serotonin-norepinephrine-dopamine releasing agent (SNDRA), with EC50 values for serotonin, norepinephrine, and dopamine release of 18.5 nM, 26.9 nM, and 8.6 nM, respectively.
Chemical and physical characteristics
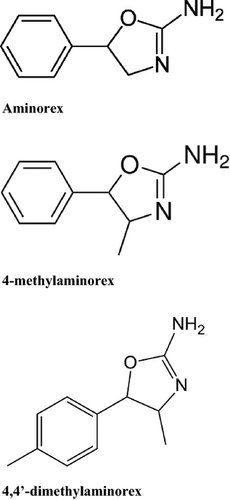
4,4′-DMAR, IUPAC name 4-methyl-5-(4-methylphenyl)-4,5-dihydrooxazol-2-amine, is a synthetic substituted oxazoline derivative classified as an analogue of both aminorex and 4-methylaminorex (fig. 1) 10. Aminorex is listed in Schedule I, and 44-DMAR/buy 44-dmar online is listed in Schedule IV of the 1971 United Nations Convention on Psychotropic Substances 10. Chemical differences among these stimulants are related to the presence of methyl groups. Aminorex has no methyl groups, while 4-methylaminorex has a 4-position methyl group located on the oxazoline ring 11. 4,4′-DMAR contains an additional methyl group in para-position on the phenyl ring 11. 4,4′-DMAR, molecular formula C11H14N2O, has two chiral centres within the oxazoline ring leading to four enantiomers 11. Cis- and trans-free base racemates prepared from the same 4′-methyl-norephedrine precursor performed with cyanogen bromide or cyanate to synthesize both products have been described as colourless solids 12. Cis- and trans-free base forms show a melting point of 136–138°C and 101–103°C, respectively, while the water-soluble cis-4,4′-DMAR hydrochloride form shows a melting point of 163–165°C 12. The cis-44-DMAR/buy 44-dmar online hydrochloride salt is a white crystalline powder available as research chemical in online research chemical stores 12.

Pharmacology
No study has investigated the effects of 4,4′-DMAR in human beings, and no pharmacokinetic studies are available. Recently, a pharmacodynamic study performed in male Sprague–Dawley rat synaptosomes has investigated the monoamine release induced by (+/−)-cis-4,4′-DMAR comparing the activity of these racemates with that of d-amphetamine, aminorex and (+/−)-cis-4-methylaminorex 12. The study showed that (+/−)-cis-44-DMAR/buy 44-dmar online, d-amphetamine, aminorex and (+/−)-cis-4-methylaminorex were potent dopamine, noradrenaline and serotonin releasers (table 1). Dose–response curve highlighted that (+/−)-cis-4,4′-DMAR elicited a potent releasing activity at dopamine (DAT), noradrenaline (NET) and serotonin transporters (SERT) with a EC50 of 8.6 (+/−) 1.1 nM, 26.9 (+/−) 5.9 nM and 18.5 (+/−) 2.8 nM, respectively (table 1). The study also demonstrated that the DAT/SERT ratio for (+/−)-cis-4,4′-DMAR, d-amphetamine, aminorex and (+/−)-cis-4-methylaminorex was 2, 473, 45 and 31, respectively 12. In another recent study performed in rat brain synaptosomes, monoamine release activity of both cis-4,4′-DMAR and trans-4,4′-DMAR isomers was compared to that of (S)-(+)-3,4-methylenedioxymethamphetamine (MDMA) 13. The study showed that cis-4,4′-DMAR and trans-4,4′-DMAR isomers exerted a dopamine- and noradrenaline-releasing activity more potent than that of MDMA (table 1). Regarding the activity at SERT, cis-4,4′-DMAR showed to be a fully efficacious releasing agent, while trans-4,4′-DMAR acted as a fully efficacious uptake blocker (table 1) 13. The study has also highlighted that the DAT/SERT ratio for (S)-(+)-MDMA, cis-4,4′-DMAR and trans-4,4′-DMAR was 0.6, 1.6 and 2.5, respectively
Toxicology
No pre-clinical or clinical study has evaluated the toxicological effects of 4,4′-DMAR in human beings. Since October 2013, 44-DMAR/buy 44-dmar onlinehas been analytically confirmed in biological samples of thirty-one deaths and one severe intoxication reported to the EMCDDA via the early warning system 11. Poland signalled a non-fatal intoxication which occurred in September 2013 and that involved a 16-year-old female admitted to the hospital after legal high consumption. She felt ill, collapsed and vomited after smoking an unknown herbal mixture. In a blood sample collected 24 hr after admission 4,4′-DMAR was found at a concentration of 0.448 mg/L 11. Regarding the thirty-one deaths (table 2), twenty-two were reported in the United Kingdom between June 2013 and June 2014, eight in Hungary between June and October 2013 and one in Poland in July 2013 11. Except for a case in the United Kingdom, sex and age data were collected for all deceased people. They were twenty-two males and eight females with a middle age of 27.93 years. In twenty-seven cases, 4,4′-DMAR was quantified in blood samples, and in three of them, it was also quantified in urine samples. In one case, 44-DMAR/buy 44-dmar online was the only detected substance, while in all the other cases, additional drugs were found, including cocaine, amphetamines, cannabis, benzodiazepines, antidepressants, second-generation antipsychotics, opioids and synthetic cathinones. Clinical examination demonstrated high body temperature, pupil dilation, muscular spasm, seizure, increased perspiration, cardiac and respiratory arrest, agitation, confusion, unconsciousness and paranoia. Autopsy revealed bleeding in muscles and organs, brain and pulmonary oedema, right atrial and ventricular dilation
Medical use
To the best of our knowledge, there is no approved medical indication for the 4,4′-DMAR, and it must be considered a drug for research and forensic utilization 11, 12. The (4S,5S)-trans-4,4′-DMAR enantiomer has been used in some patients related to the preparation of anti-inflammatory medicines based on phospholipase A2 inhibitors 15.
Analytical detection
To date, there is no immunoassay field test for the detection of 4,4′-DMAR in biological samples. Furthermore, there is no information about potential cross-reactivity with other common drugs of abuse. In addition, there is no information on presumptive colour tests with 44-DMAR/buy 44-dmar online. A recent case series study performed on eighteen individuals who died after 4,4′-DMAR consumption found that gas chromatography–mass spectrometry (GC-MS) screening proved relatively insensitive while satisfactory results were obtained by liquid chromatography–mass spectrometry (LC-MS), high-resolution LC-MS and ultra-high-performance liquid chromatography with diode array detector (U/HPLC-DAD)
Legality
The UK Home Office expressed intent to ban 4,4′-DMAR following advice from the Advisory Council on the Misuse of Drugs and subsequently it became a class A drug on 11 March 2015.
4,4′-DMAR is an Anlage II controlled substance in Germany as of May 2015.[8]
Sweden’s public health agency suggested to classify 4,4′-DMAR as hazardous substance on November 10, 2014.
4,4′-DMAR is also banned in the Czech Republic